
The color of a precipitate is one clue to its identity. The reaction between cadmium sulfate and potassium sulfide in water forms potassium sulfate and cadmium sulfide.ĬdSO 4(aq) + K 2S(aq) ⟶ K 2SO 4(aq) + CdS(s).
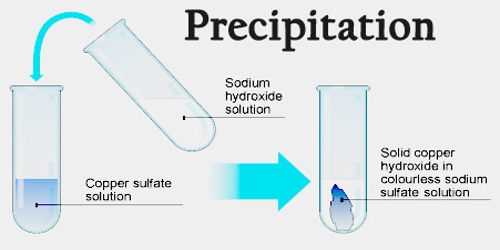
The reaction between sodium sulfate and strontium chloride forms sodium chloride and strontium sulfate, which is a precpitate.Reacting copper sulfate and sodium hydroxide forms sodium sulfate and copper hydroxide.NaF(aq) + AgNO 3(aq) ⟶ AgF(s) + NaNO 3(aq) (molecular) Reaction between sodium fluoride and silver nitrate in water, forming solid silver fluoride and aqueous sodium nitrate:.Pb 2+(aq)+2I −(aq)⟶PbI 2(s) (net ionic equation) Reaction between potassium iodide and lead nitrate in water, forming lead iodide as a precipitate and aqueous potassium nitrate:ĢKI(aq) + Pb(NO 3) 2(aq) ⟶ PbI 2(s) + 2KNO 3(aq) (molecular equation).Compare the different ways to write the reactions. Note the way precipitation reactions appear as molecular equations and net ionic equations. Here are common examples of precipitation reactions. When the particles become large enough, they precipitate or fall out of solution. Initially, nucleation may lead to the formation of a suspension of tiny solid particles in liquid. Other nucleation sites include solid impurities in the solution and gas bubbles. During nucleation, tiny particles adhere to each other and to surface imperfections on the container. In all cases, precipitation starts with nucleation. The other ways of forming precipitates are more processes than reactions.

Adding ions is another option, driving a compound toward solidification.Ī double replacement reaction that forms a precipitate is a precipitation reaction. Changing the solution: Introducing a new solvent in which a chemical is insoluble often causes precipitation.Controlling temperature and pressure causes chemicals to precipitate out of solution. Particles aggregate during the nucleation stage and the substance falls out of solution until equilibrium is reached. Crystallization: Even in a pure solution, concentration often exceeds solubility.The dissolved salts react and one or more of the products is insoluble (or at least partially insoluble). Double replacement reaction: Often, precipitation results from a double replacement reaction between two aqueous solutions.Precipitation results from the concentration of a chemical exceeding its solubility. Down arrow: Otherwise, a down arrow (↓) after the name or formula indicates a precipitate.State of matter symbol: Including the symbol (s) following a chemical formula means the product is a solid.There are two common ways to indicate precipitation in a chemical reaction. The remaining solution is the supernate or supernatant. In chemistry, a precipitation reaction is a chemical reaction between two dissolved substances that forms one or more solid products. ".This entry was posted on Augby Anne Helmenstine (updated on February 3, 2022)Ī precipitation reaction occurs when two dissolved substances react and form one or more solid products. Inglethorp was the identical strychnine prescribed by Dr. "Do you mean that the murderer introduced the strychnine into her tonic?" Captain Hastings cried. When the victim took the final dose of medicine, it contained a lethal dose of transparent strychnine bromide salt. This salt precipitated and fell to the bottom of the bottle.
The murderer added potassium bromide to the medicine, which reacted with the strychnine sulfate to form strychnine bromide, a transparent insoluble salt. The strychnine in the medicine was in the form of strychnine sulfate. However, if all the strychnine in the bottle were taken at once, it would be fatal! There was too little strychnine in a single dose to cause harm. The victim was taking medicine that contained a small amount of dissolved strychnine. A precipitate was famously and cleverly the murder method used by Agatha Christie in her first novel The Mysterious Affair at Styles, which features strychnine poisoning.


 0 kommentar(er)
0 kommentar(er)
